MOLECULAR SIEVE DESCRIPTION
Zeolite molecular sieves are crystalline microporous materials with a uniform pore structure. They function as “molecular sieves” by selectively adsorbing molecules small enough to pass through their pores, while excluding larger ones—enabling precise separation by size and shape.
The framework is composed of SiO₄ and AlO₄ tetrahedra interconnected via oxygen atoms, forming a well-defined three-dimensional network with molecular-scale cavities and consistent pore channels.
Thanks to their structural diversity, exceptional hydrothermal stability, and unique selectivity, zeolite molecular sieves are widely used in petroleum refining, gas separation, catalysis, and other industrial processes—where they play an essential role in enhancing efficiency and enabling key technologies.
[Application]
Wood products, automobile, shipbuilding, home appliances, container production factory spray paint, painting workshop of organic waste gas purification, can also be used in shoes, rubber, printing, chemical plastics, cable, enameled wire and other assembly line supporting equipment use
ADVANTAGES OF MOLECULAR SIEVES
1.GOOD HYDROTHERMAL STABILITY :Exhibits significant adsorption capacity even under relatively high temperatures and high humidity conditions.
2.PRECISE SELECTIVITY:Its uniform internal pore size distribution enables effective molecular recognition, thereby significantly enhancing the molecular sieve's selective adsorption capacity for VOCs molecules.
3.DESORPTION DIVERSITY:As a non-flammable material, it allows for desorption through various methods such as pressure swing, microwave, and hot air desorption.
4.HIGH ADSORPTION CAPACIT:The material possesses a highly porous internal structure and an extensive specific surface area, enabling it to adsorb contaminants hundreds of times its own volume.
5.TUNABLE HYDROPHOBICITY:The hydrophilicity/hydrophobicity of the molecular sieve can be adjusted by modifying its framework silicon-to-aluminum ratio. Molecular sieves with a high silicon-to-aluminum ratio exhibit excellent hydrophobic properties, effectively reducing competitive adsorption of water against VOC molecules under certain humidity conditions.
6.REVERSIBILITY:It exhibits reversible adsorption of both liquids and gases.
Desorption Advantage — Customer Demonstration



Before Desorption: Visible accumulation of dust particles adhered to the molecular sieve surface.
After High-Temperature Desorption: The molecular sieve surface is clean with little to no adhered substances.This demonstrates effective treatment of inadequately pretreated waste gas, with desorption restoring the molecular sieve's original performance.
Desorption Advantage — Visual Results of High-Temperature Regeneration


Before Desorption: The molecular sieve appears yellow with black traces, visibly saturated with adsorbed organic compounds and oil stains.
After High-Temperature Desorption: The surface is clean and free of attachments, indicating thorough regeneration.This process effectively handles inadequately pre-treated exhaust gas or incomplete on-line desorption, fully restoring the molecular sieve's original performance after regeneration.
PRODUCT APPLICATION CONDITIONS
Application condition | ||
Best applicable conditions | Suitable for organic components | Organic components are not applicable (Except for a special molecular sieve) |
Low concentration Wind volume Humidity<60% temperature<50℃ Recommended empty tower flow rate: 1m/s Recommended loading height: 700mm | Benzene series (benzene, toluene dimethylbenzene) Alcohol, ketones, aldehydes Part of the ester alkane |
hardener Polymers are prone to occur halogen heavy metallic salt |
PRODUCT ADSORPTION CAPACITY TABLE
VOCs name | Adsorption capacity (KG/m ') | VOCs name | adsorption capacity (KG/m') |
benzene | 16 | acetic ether | 29 |
methylbenzene | 18 | butyl acetate | 27 |
dimethylbenzene | 14 | acetic acid | 45 |
styrene | 20 | alcohol | 14 |
acetone | 15 | butanone | 17 |
cyclosiloxane | 17 | isopropanol | 20 |
Propylene glycol monomethyl ether | 17 | n-butyl alcohol | 20 |
Propylene glycol and methyl ether acetate | 20 | Diethylene glycol butyl ether | 18 |
1. Test conditions: concentration of 300-1000mg/m³, residence time of 0.55s/m, outlet of 20-50mg/m³.
2. The data of laboratory tests is only for reference, and the adsorption amount is related to the working conditions and emission standards, and the adsorption amount will increase after enlarging the working conditions.
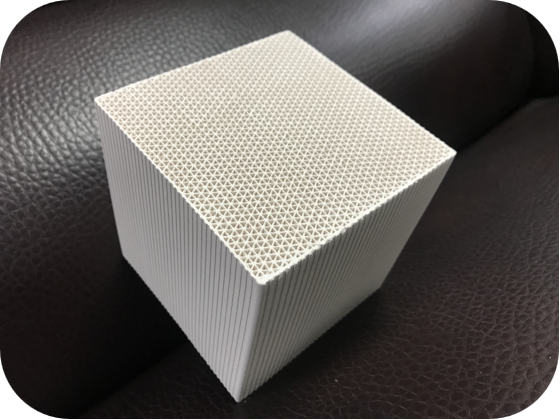
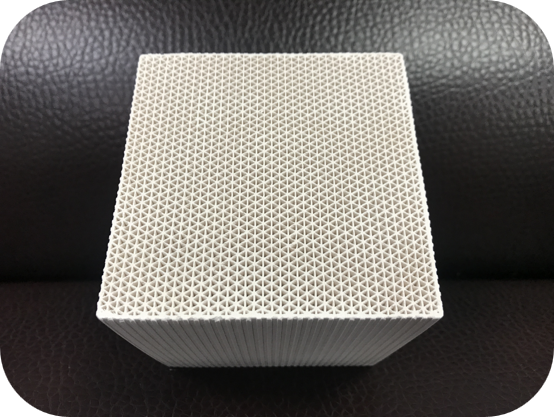
TECHNICAL PRODUCT SPECIFICATIONS
project | function |
Overall dimensions (mm) | 100*100*100 |
hole count (cm²) | 16-25 |
Hole wall thickness (mm) | 0.4-0.5 |
Longitudinal resistance strength (Mpa) | 0.7 |
Transverse resistance strength (Mpa) | 0.2 |
Density (g/cm ³) | 0.31-0.43 |
Specific surface area (m²/g) | <900 |
purifying rate (%) | >90% |
end-use temperature (℃) | <600 |
Adsorption wind speed (m/s) | 0.8-1.2 |
Adsorption residence time (s/m) | 0.6-0.9 |
Detachment wind speed (m/s) | >0.3 |
Desorption temperature: (℃) | >200 |